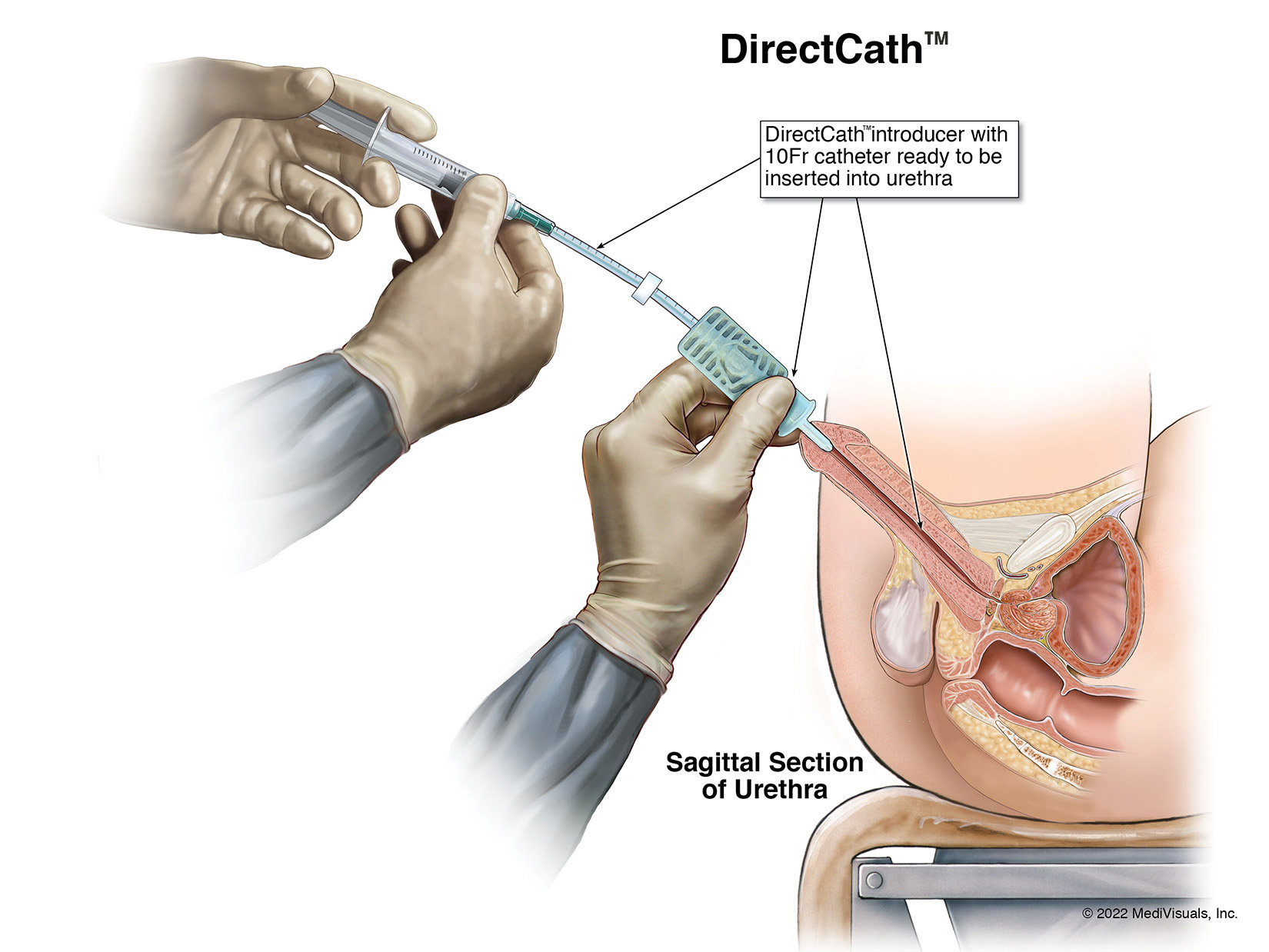
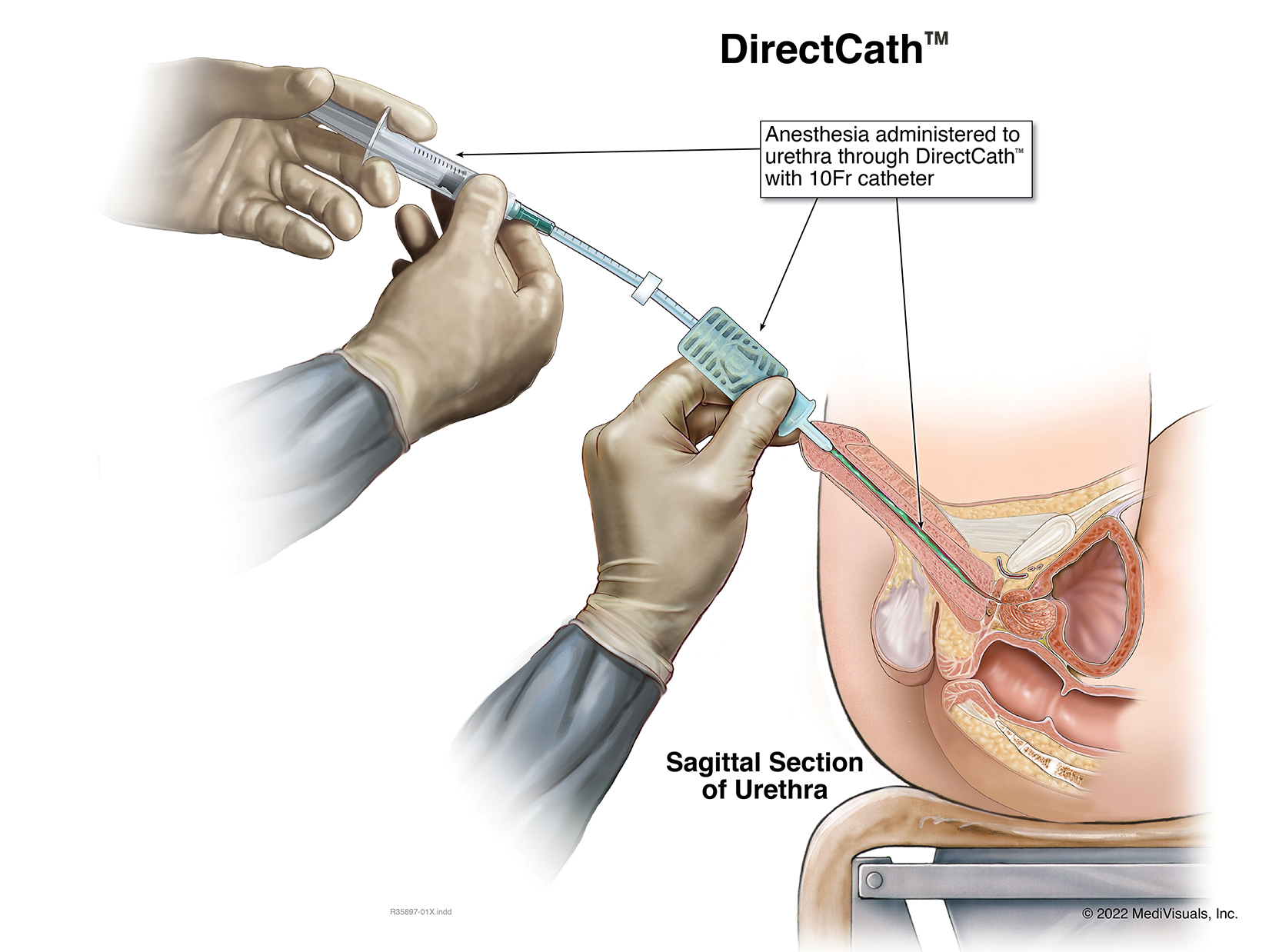
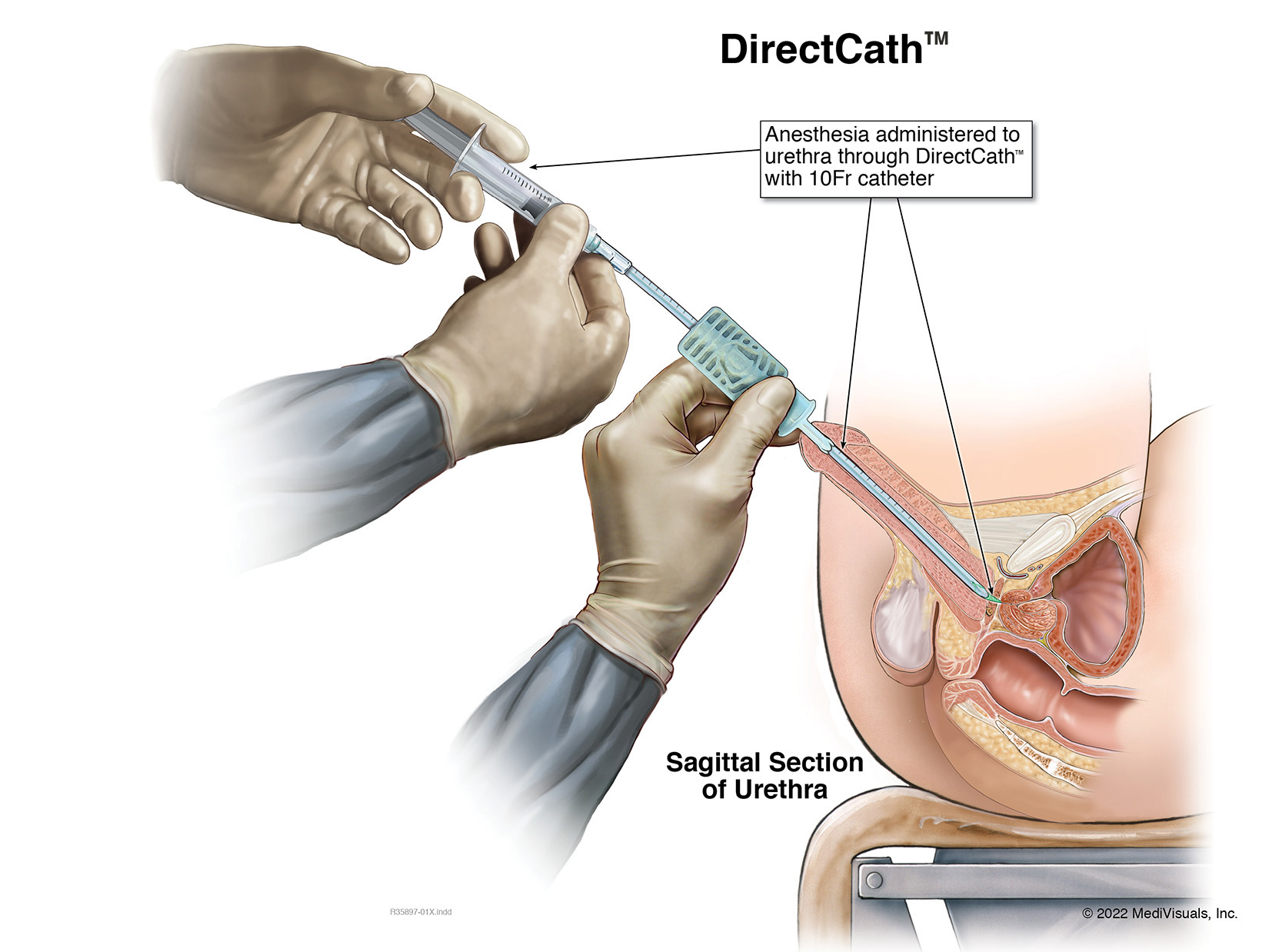
The Next Generation Prep for Urological Examinations USPTO #: US 2021-0299410 A1


Demographics
- As of Q-3 2021 over 19,000 urologists were “actively practicing” in the USA.
- The average urologist performs ten (10) in-office urological examinations/day.
- Based on a 4-day work week, 48 work weeks equals 36,480,000 exams/year.
- The majority of these exams will require a method to anesthetize the urethra.
- Uniquely enough, the DirectCath™ thoroughly anesthetizes the urethra from the glans to the neck of the bladder otherwise known as the urethral sphincter.
- Designed to reduce the onset of pain associated with a urological examination.
- Less patient stress can improve a practitioner’s confidence.
- Positive patient and procedural outcomes yield more precise diagnoses.
- No other device like the DirectCath™ is available on the market world-wide.
- DirectCath™ is designed to improve how an in office urological exam is prepped.
Background Rationale
The commercially available methods of anesthetizing the urethra are as follows: Oral Tip Syringe, Modified Catheter Tip Syringe, or 5gm Aluminum Tube.
- Glydo® (lidocaine HCl jelly USP) 2% – Sagent Pharmaceuticals (oral)
- Urojet® (lidocaine HCl jelly USP) 2% – Amphastar Pharmaceuticals (modified)
- AKORN (lidocaine HCl jelly USP) 2% – AKORN Pharmaceuticals (tube – 5g & 30g)
- The practitioner places the syringe tip into the glans all the way to syringe neck.
- A positive seal must be made by pressing the glans tight to the neck of syringe.
- Lidocaine is then dispensed by pushing on the syringe piston so that it drives the anesthetic as far into the urethra as possible, thus inducing extreme pain.
The DirectCath™ is designed to provide a thorough urethral anesthetization from the glans past the neck of the bladder also known as the urethral sphincter.
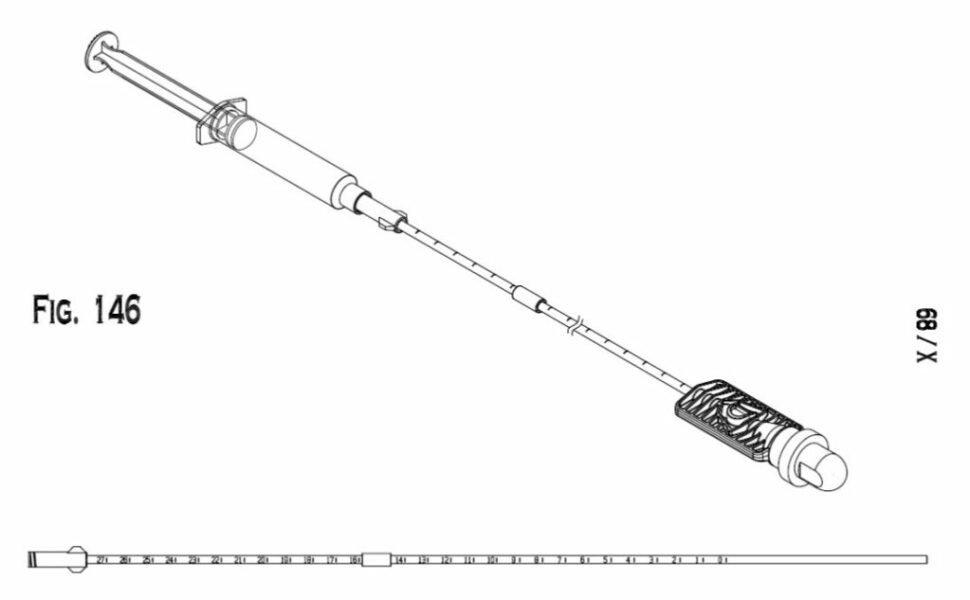
The DirectCath™ Anesthetizing Device
These are the CPT Codes that commonly use Lidocaine for an in-office setting:
- 52000 Cystourethroscopy
- 52005 Cystourethroscopy with retrograde urography
- 52204 Cystourethroscopy, with biopsy
The ability to irrigate the urethra with an anesthetic from the glans to the bladder sphincter is a first of its kind device for the urological market world-wide; this is one of the patented concepts of the device.
- The DirectCath™ is designed to work in conjunction with the following products:
- Glydo® (lidocaine HCl jelly USP) 2% – Sagent Pharmaceuticals (oral tip)
- Urojet® (lidocaine HCl jelly USP) 2% – Amphastar Pharmaceuticals (modified tip)
- AKORN (lidocaine HCl jelly USP) 2% – AKORN Pharmaceuticals (tube – 5g/30g)
- 2% Buffered Lidocaine Solution – Prepared by the practitioner.
Ancillary Study - Harvest “fresh” urine for cellular evaluation
- It was suggested that the DirectCath™ may provide a clean urine sample which currently is contaminated when using the UroJet method to anesthetize the urethra ahead of a procedure.
- Harvest a urine sample via the DirectCath™ Anesthetizing Device and evaluate
- Sample Quality = clarity (blood, debris/gel, etc.) via analytical turbidity test.
- Harvest a urine sample via the UroJet™ System Device and evaluate
- Sample Quality = clarity (blood, debris/gel etc.) via analytical turbidity test.
- New research to monitor bladder cancer has been developed by evaluating specific cells excreted in the urine; we believe the DirectCath™ may be superb to harvest a fresh urine sample.
Features & Benefits
- Designed to reduce the onset of pain associated with an urological examination.
- Less patient stress can improve a practitioners confidence.
- Positive patient and procedural outcomes yields more precise diagnosis’s.
- DirectCath™ is designed to improve how an in office urological exam is prepped.
The only known commercially available method of anesthetizing the urethra is using an oral tip (Glydo®) and/or a modified catheter type tip (UroJet™) syringe.
- Glydo® (lidocaine HCl jelly USP) 2% – Sagent Pharmaceuticals
- Urojet® (lidocaine HCl jelly USP) 2% – Amphastar Pharmaceuticals
- **The DirectCath™ device does not require a penile clamp.
- **Complete anesthetization can be achieved in less than 5 minutes.
- The concept of the DirectCath™ is based on a closed system catheter.
- The primary difference is this device will be used by professionals in an office setting, trained on clean and sterile technique; the DirectCath™ Anesthetizing Device is placed into the glans, by means of a silicone introducer tip.
- The introducer tip is designed protect the DirectCath™ catheter from being contaminated yielding a safe trek past the region of the urethra commonly known to be a repository for a wide variety of pathogenic bacteria.
- The catheter tube of the DirectCath™ is fitted with centimeter graduations to record the depth that the tube tracked and the distance the medication was dispensed; this is a novel concept of the device.
- As the catheter pushes forward the anesthetic can immediately begin to be dispensed, tracking its way through the urethra to the sphincter.
The concept design is to thoroughly anesthetize the urethra.
- The DirectCath™ Anesthetizing Device works in conjunction with a luer lock syringe to slowly dispense a medication, anesthetic solution and or gel.
- Patient studies have determined that the majority of pain experienced with a urological examination occurs when approaching to and or passing an object through the urethral sphincter also known as the neck of the bladder.
- The DirectCath™ is designed to provide the patient a pain reduced examination giving the urologist an unrestricted free access into the bladder.
- The ability to irrigate the urethra with an anesthetic from the glans to the bladder sphincter is a first of its kind device for the urological market world-wide; this is one of the patented concepts of the device.
Medical Drawings
Clinical Study
DirectCath™ Anesthetizing Device Phase 1 - 100 men will be anesthetized with the DirectCath™ Device to access the level of pain experienced while being anesthetized.
- The patients will be identified by his unique barcoded wrist band and barcoded chart as a new patient (NP) or returning patient (RP).
- The patient will be anesthetized using a 1% or 2% Lidocaine Buffered Solution via luer lock syringe
- The depth that the anesthetic was delivered into the urethra will be recorded by centimeter graduations as referenced on the catheter itself.
- The practitioner will have limited or no knowledge of the subjects past history
- The patient will assess pain utilizing the Numeric Rating Scale (NRS)
UroJet™ Modified Syringe Phase 1 - 100 men will be anesthetized with the UroJet™ Device to access the level of pain experienced while being anesthetized.
- The patient will be identified by his unique barcoded wrist band and barcoded chart as a new patient (NP) or returning patient (RP).
- The patient will be anesthetized using the 2% Lidocaine Gel via UroJet modified syringe as packaged by the manufacturer.
- The practitioner will have limited or no knowledge of the subjects past history
- The patient will assess pain utilizing the Numeric Rating Scale (NRS)
Phase 2 - The level of pain experienced during the actual procedure.
- Patients whereby the DirectCath™ Anesthetizing Device was used
- The patient will be identified by his barcoded wrist band and chart
- The patient will then be assessed for the amount/value of pain experienced during the procedure utilizing the Numeric Rating Scale (NRS)
- Patients whereby the UroJet™ Modified Syringe was used
- The patient will be identified by his barcoded wrist band and chart
- The patient will then be assessed for the amount/value of pain experienced during the procedure utilizing the Numeric Rating Scale (NRS)
Phase 3 – Harvest a fresh urine sample for cellular evaluation - (Ancillary Study)
It has been suggested that the DirectCath™ may even more useful than originally projected because the design concept could provide a clean urine sample for use in monitoring the progression and/or remission of bladder cancer.
Rationale: When the UroJet/Glydo method is used to anesthetize the urethra, the urine sample is contaminated with epithelial cells.
Hypothesis: Excessive stress is placed into the urethra due to the pressure required to push the anesthetic (lidocaine gel) to properly anesthetize the urethra .
- Harvest a urine sample via using the DirectCath™ Anesthetizing Device
- Sample Quality = clarity (blood, debris/gel, etc.) via analytical turbidity.
- Harvest a urine sample via the UroJet™ System Device and evaluate
- Sample Quality = clarity (blood, debris/gel etc.) via analytical turbidity.